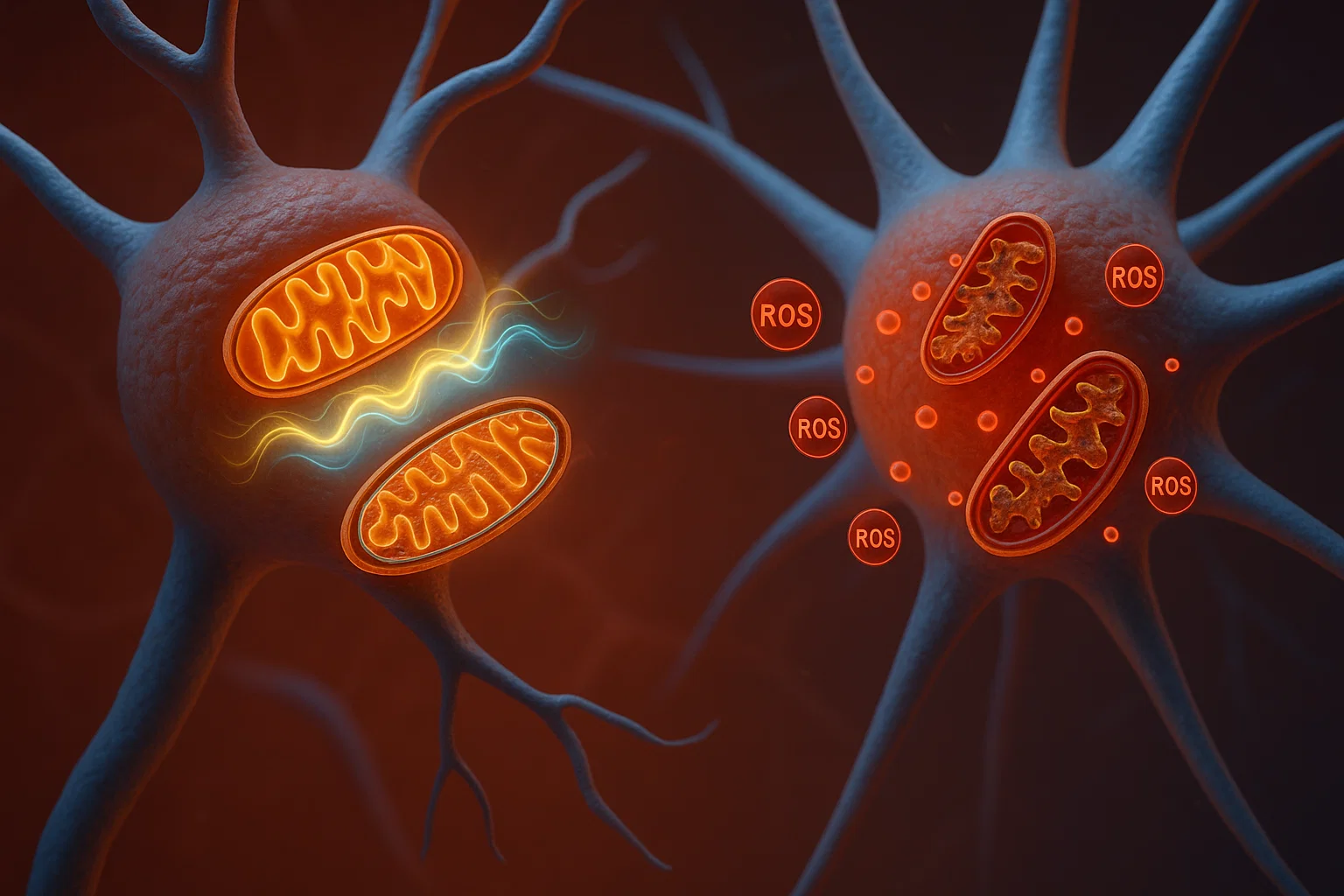
Neurological conditions, including stroke, Alzheimer’s, Parkinson’s, and many others, affect millions worldwide and represent a leading cause of death and disability. These disorders often share a common underlying problem: mitochondrial dysfunction. Mitochondria, the energy-producing “powerhouses” of cells, play a crucial role in brain health. When they malfunction, it can trigger oxidative stress, inflammation, and cell death, accelerating disease progression.
Current treatments for these diseases primarily manage symptoms but do not prevent or reverse mitochondrial damage. This gap creates an urgent need for neuroprotective therapies that can safeguard or restore mitochondrial function.
What Are Transition Metal Dichalcogenide Nanoflowers?
Transition metal dichalcogenides (TMDs) are a class of nanomaterials composed of layers of transition metals combined with sulfur or selenium atoms. When these TMD sheets self-organize into flower-like nanostructures, they are called nanoflowers (NFs). Two notable examples studied recently are molybdenum disulfide (MoS₂) and molybdenum diselenide (MoSe₂) nanoflowers.
These nanomaterials have unique physical and chemical properties, such as a very high surface area-to-volume ratio, making them promising for various applications, including electronics, catalysis, and biomedical uses.
The New Study: Neuroprotective Effects of MoS₂ and MoSe₂ Nanoflowers
Researchers at Texas A&M University investigated the biological effects of MoS₂ and MoSe₂ nanoflowers on brain cells—specifically neurons and astrocytes, which are vital for brain function and repair. Their study found compelling evidence that these nanoflowers can protect brain cells by:
Reducing reactive oxygen species (ROS) harmful molecules that damage cells.
Decreasing mitochondrial impairment improving mitochondrial health and function.
Increasing mitochondrial biogenesis promoting the creation of new mitochondria.
Extending lifespan in a living model organism specifically, Caenorhabditis elegans (a tiny worm used in biological research).
How Do These Nanoflowers Protect Brain Cells?
The key to the neuroprotective effects lies in activating a specific biological pathway involving PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1 alpha). This pathway controls mitochondrial biogenesis and regulates oxidative stress.
When neurons and astrocytes were treated with MoS₂ and MoSe₂ nanoflowers, the researchers observed:
A significant decrease in ROS levels, protecting cells from oxidative damage.
A dramatic reduction in mitochondrial damage measured through specific cell assays.
An upregulation of PGC-1α and related mitochondrial genes, leading to healthier mitochondria.
An increase in proteins essential for mitochondrial function like succinate dehydrogenase and cytochrome c oxidase.
These changes collectively support improved energy production and cellular health in brain cells.
Lifespan Extension in Model Organisms
The study went beyond cell cultures and tested the effects on C. elegans, a widely used model organism in aging and neurological research. The worms treated with MoSe₂ nanoflowers showed an impressive increase in lifespan up to 6 days longer than untreated worms, which is significant given their short natural lifespan.
This result supports the idea that TMD nanoflowers can enhance mitochondrial health in living organisms, potentially translating into therapies that slow neurodegeneration and aging in humans.
Why Is This Research Important?
Neurological disorders are notoriously difficult to treat, and mitochondrial dysfunction is a major barrier in developing effective therapies. This study presents TMD nanoflowers as a novel class of neuroprotective agents that directly target mitochondrial health, opening new therapeutic avenues.
Because mitochondria regulate so many cellular functions—from energy metabolism to apoptosis (cell death) improving their function could:
Slow or halt progression of neurodegenerative diseases.
Reduce brain inflammation and oxidative damage.
Potentially extend healthy lifespan and cognitive function.
Next Steps and Future Research
While this study demonstrates promising neuroprotective effects in vitro (cell cultures) and in vivo (worms), further research is needed to:
Understand the precise mechanisms by which TMD nanoflowers interact with brain cells.
Determine optimal nanoflower size, shape, and composition for maximal benefits.
Test safety and efficacy in mammalian models.
Develop delivery systems to target these nanomaterials to the human brain.
Conclusion
The discovery that transition metal dichalcogenide nanoflowers, especially MoS₂ and MoSe₂, can protect brain cells by enhancing mitochondrial function marks a breakthrough in neurodegenerative disease research. These nanomaterials offer a promising path toward effective neuroprotective treatments that could improve quality of life for millions suffering from conditions like Alzheimer’s and Parkinson’s disease.
As research progresses, we may soon see nanotechnology play a pivotal role in protecting brain health and combating some of the most challenging neurological disorders.
Reference
Mitchell, C. L., Matveyenka, M., & Kurouski, D. (2025). Neuroprotective properties of transition metal dichalcogenide nanoflowers alleviate acute and chronic neurological conditions linked to mitochondrial dysfunction. Journal of Biological Chemistry.