Millions of people are affected by parasitic infections and one of the most widespread skin penetrating helminth entails schistosoma mansoni as a major cause of chronic schistosomiasis. Although its incursion can be easily overlooked, a recent study has, surprisingly, come up with an answer to help in our defense mechanism: pain sensing neurons. In particular, TRPV1+ neurons that are sensitive to heat and capsaicin (“hot” of chili peppers) are now seen to be important in activating immune responses to prevent parasite infections and their dissemination throughout the system.
TRPV1 Neurons: More Than Just Pain Sensors
Not only is the skin a physical barrier but it is also a center of immune and sensory response. The Transient Receptor Potential Vanilloid 1 ion channel better known as TRPV1+ neurons helps to mediate both pain and heat sensation in the body. Part of the effect however, is due to the release of immune-modulating neuropeptides such as CGRP and Substance P by these neurons when stimulated.
TRPV1+ neurons play both inhibitory and stimulatory roles in the immune responses in bacterial and fungal infection. The most recent studies examine the possibility of having compatible mechanisms in combating S. mansoni.
A mute Parasite and a muffled Defence
S. mansoni invades unnoticed as opposed to instances seen with other skin infections characterized by the inflammatory nature of the skin or discomfort. Researchers revealed that when the parasite gets into the skin it actively inhibits the responses of TRPV1+ neurons. S. mansoni infected mice exhibited decreased heat pain sensitivity and a reduced influx of calcium following heat stimulus into the DRG (dorsal root ganglia) nerve cells, which is indicative of inhibited TRPV1 activity. This gave the parasite a way to evade rapid immune detection.
Unexpectedly, despite still retaining a portion of TRPV1 in these neurons, functional responses to capsaicin were dampened- probably by a product of the parasite.
Turning On Pain to Strengthen the Immune System
In order to valid whether reactivation of TRPV1+ neurons would restore immune defense, the researchers stimulated the neurons with optogenetics method and exposed the activated neuros to S. mansoni. The impressions were dramatic:
Penetration of parasites through the skin was greatly reduced.
There were less parasites in the lungs.
Engagement of TRPV1+ neurons increased the expression markers of immune activation IL-17, IL-13 and Ki-67 in both 3T T cells and CD4+ helper T cells.
Recruitment of neutrophils and monocytes that expresses iNOS was greatly activated.
This evidence can be used to follow the hypothesis that pre activating the TRPV1+ neurons prior to exposure can prim the immune defenses of the skin.
So What Occurs, When TRPV1+ Neurons Are Disabled?
To interpret the reverse event, the team chemically destroyed TRPV1+ neurons with the resiniferatoxin (RTX). This silencing and the consequent:
More parasite load to the lungs.
Cytokine and skin gamma delta T-cell proliferation.
There was a reduction in neutrophils and monocytes that come to the infection region.
These findings strengthen the notion that TRPV1+ cells play a role in inducing a fast, localized immune response that prevents or forces minimum dissemination of the parasite in the organism.
The Bigger Picture: Pain, Immunity and Parasites
This work falls into a developing field known as neuroimmunology the study of communication between the nervous and the immune systems. It proposes that some pathogens, such as S mansoni, have developed the means of hijacking or silencing sensory neurons as a means of avoiding detection. In the current example, the parasite prevents the activation of TRPV1+ neurons to evade the use of IL-17/neutrophil pathways, which play key roles in skin mediated immune responses.
Interestingly, capsaicin-driven TRPV1 activation in a topical manner has already been demonstrated to induce cytokines such as IL-17 and IL-22. This is opening up future prospects with regards to application of TRPV1 targeting interventions such as creams or light therapy as a means to bolster immunity in skin infections.
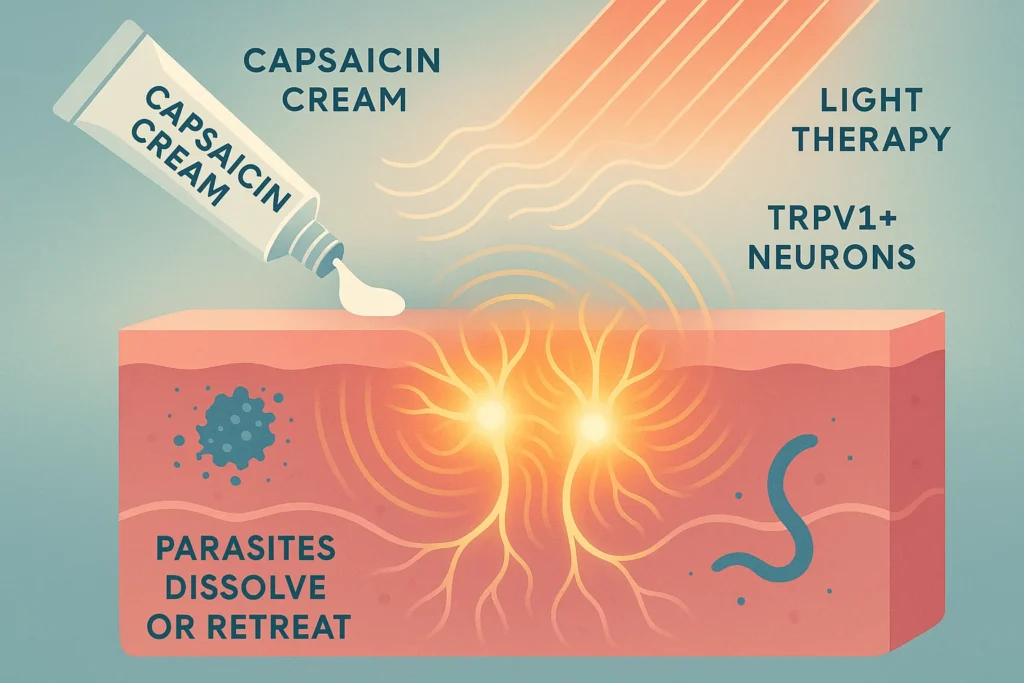
Conclusion: Are our nerves trainable to fight parasites?
In this study, TRPV1+ sensory neurons have been found not only to be passive pain sensors, but play an immune defense role as well. Stimulating these neurons, we could come up with new treatments which would prevent or treat parasitic diseases such as schistosomiasis. On the other hand,How pathogens inhibit these neurons might provide an idea of how pathogens evade immunity.
With such precision medicine, merging neurobiology with immunology can transform against fighting infectious diseases in the future.
References and Resources:
Inclan-Rico, J. M., et al. (2025). “TRPV1+ neurons promote cutaneous immunity against Schistosoma mansoni.” The Journal of Immunology, https://doi.org/10.1093/jimmun/vkaf141 .
National Institute of Allergy and Infectious Diseases – Schistosomiasis Resource Center
Jackson Laboratory Mouse Models – TRPV1Cre and Ai32 strains