Wearable health technologies have already transformed how people track conditions such as diabetes, fitness and heart health. One of the best known success stories in this space are continuous glucose monitors (CGMs), which are life saving devices that deliver millions of users with a continuous glucose reading. But glucose is only one biomarker, and the future of personalised medicine depends on the finding of new, less invasive ways to monitor a far wider range of biochemical signals.
A new study in Lab on a Chip describes a self powered microneedle (MN) microfluidic platform for sampling interstitial fluid (ISF) a body fluid that directly correlates to the blood chemistry. This device is a significant step towards wearable, minimally invasive and economical diagnostic systems.
Why Interstitial fluid is important
Interstitial fluid the liquid surrounding and nourishing cells. As a medium of exchange between blood capillaries and tissues, it has a basically identical composition with that of blood. Many studies have found that 90-99% of proteins in blood occur in ISF, and other novel biomarkers not present in blood
Unlike sweat which has been the focus of wearable diagnostics before but contains only small amounts of proteins, and suffers from delays, ISF offers real-time, clinically relevant information. This makes ISF a perfect candidate for next generation biosensing platforms.
The Problem of Accessing ISF
The difficulty in accessing ISF is, even though it is very valuable, the problem. Traditional methods such as suction blistering and microdialysis are invasive, painful and not suitable for regular or wearable use. This has retarded the development of ISF based diagnostic devices.
Enter microneedles: sub-millimeter structures usually 300-1000 mm long, engineered to just pierce the surface of skin without injuring nerves or blood vessels. By targeting the dermis, the microneedles can painlessly access ISF reservoirs.
How the New Microneedle System Works
The research team developed a fully passive microneedle platform that consists of three key components:
Hydrogel forming microneedles
Made of hyaluronic acid that is methacrylated, these needles absorb ISF and expand into the skin when injected without degradation.
Osmotic pump
The hydrogel with a glycerol or glucose impregnation produces negative pressure, which draws fluid into the system without having to be powered with electricity or external pumps.
Paper microfluidics
Filter paper based microfluidic channels are used to transport the extracted fluid, to conserve the biomarker and can be integrated with biosensors for analysis.
This system achieves zero power ISF extraction, so it is simple, low weight, and suitable for wearable health monitoring.
Testing the Product: Cortisol Testing
To show how the device can be used in the real world, the researchers tested it with cortisol, a stress hormone important for metabolism, immune response and mental health.
Short-term sampling (15-45 minutes): Cortisol was readily recovered in physiologically relevant concentrations demonstrating the potential of this system to deliver rapid results.
Extended sampling (24 hours): Although analyte degradation somewhat reduced recoveries, the system was still able to detect high cortisol levels, suggesting the possibility of semi-quantitative long-term monitoring
The first demonstration of cortisol recovery by means of a microneedle extraction system opens the way for stress monitoring and endocrine diagnostics outside of the clinical setting.
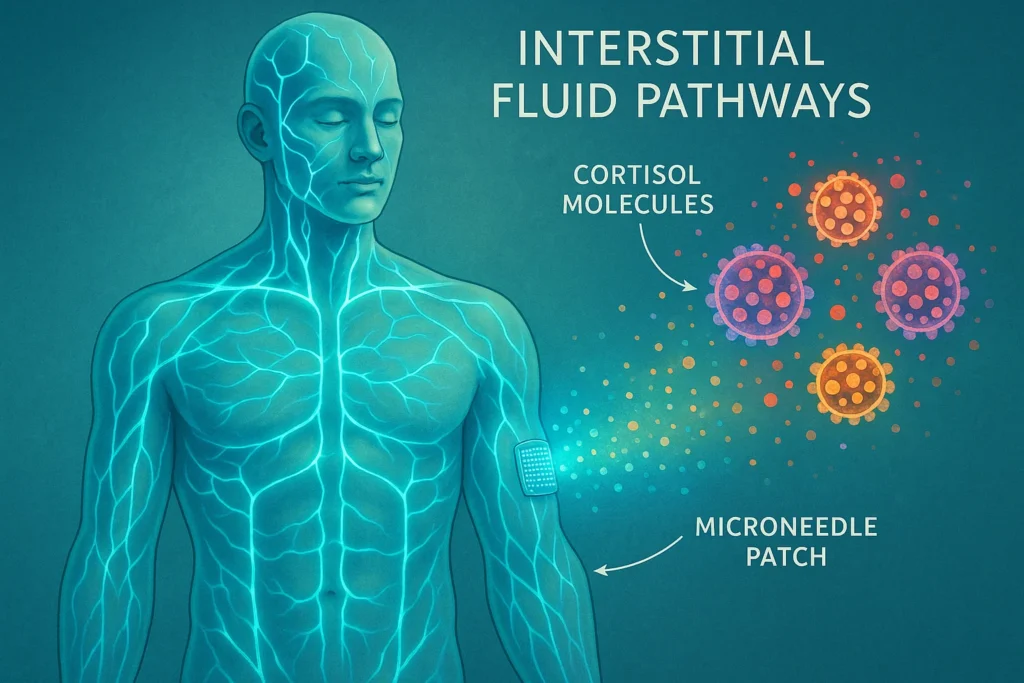
Key Advantages of the System
Minimally invasive & painless: Microneedles avoid nerves and blood vessels
Zero power consumption: Does not use batteries; no electronics detected; relies on osmotic pressure.
Low cost & scalable: Made of biocompatible polymers and paper.
Quick results: Biomarker recovery time in 15 minutes
Wearable and easy to use: Can be embedded in patches or smart bandages
These characteristics make the device very attractive for point-of-care (PoC) diagnostics and wearable health devices.
Limitations and Next Steps
While the results are encouraging, the study was done in agarose and synthetic skin models and not yet in humans. Future studies will need to answer:
Variability in actual skin (elasticity, surface texture).
More biomarkers than cortisol
Analyte stability under long term monitoring
Integration with in line biosensors for continuous measurement.
The researchers say that placing biosensors closer to the extraction site will help improve the accuracy of diagnosis, particularly for short term monitoring.
Implications for Health Care more widely
If adapted to clinical and consumer uses, this microneedle platform has the potential to revolutionize healthcare by allowing:
Stress and mental health monitoring with realtime cortisol levels
Monitoring of chronic diseases such as diabetes, cardiovascular, and disorders of the hormones.
Personalized medicine based on wearable platforms that monitor multiple biomarkers in real time.
Minimized laboratory blood draws by decentralized diagnostics
By removing the pain, complexity and expense from current ISF extraction techniques, the technology may well make at-home biochemical monitoring as ubiquitous as wearable fitness monitors.
Conclusion
The use of ISF extraction by means of a self-powered microneedle microfluidic system is a milestone in wearable diagnostics. Its passive, painless, affordable, and biomarker monitoring capabilities (cortisol) are enormous when it comes to stress management, chronic disease monitoring, and personalized health care.
As researchers proceed toward the human trials and biosensor integration, this technology has the potential to help usher in a new era of continuous, noninvasive health monitoring taking the lab to the skin.
References:
Sharkey, C. T., Aroche, A. F., Agusta, I. G., et al. Design and characterization of a self-powered microneedle microfluidic system for interstitial fluid sampling. Lab on a Chip (2025). DOI: 10.1039/d5lc00590f
Bandodkar, A. J., et al. Annual Review of Analytical Chemistry, 2019.

