Various methods have evolved by the viruses to survive in the body of human beings but there are those that are a master at concealing. According to an article published in Nature Microbiology (June 2025) a tricky ploy employed by Human T-cell Leukemia Virus type 1 (HTLV-1), a retrovirus that infects up to 5 million people and is capable of causing leukemia and nervous disorders, has been identified. Inside the virus, a research team discovered a small genetic piece that causes itself to turn off the expression of viral genes: a viral silencer element.
This silencer, in liaison with a host cell protein known as RUNX1, ensures that HTLV-1 lies dormant in infected cells protecting them against immune system detection over years, or even decades. Not only does the discovery shed light on a decades-old enigma involving how HTLV-1 latency works, but it also can provide indications that have the potential to transform the course of HIV study and management.
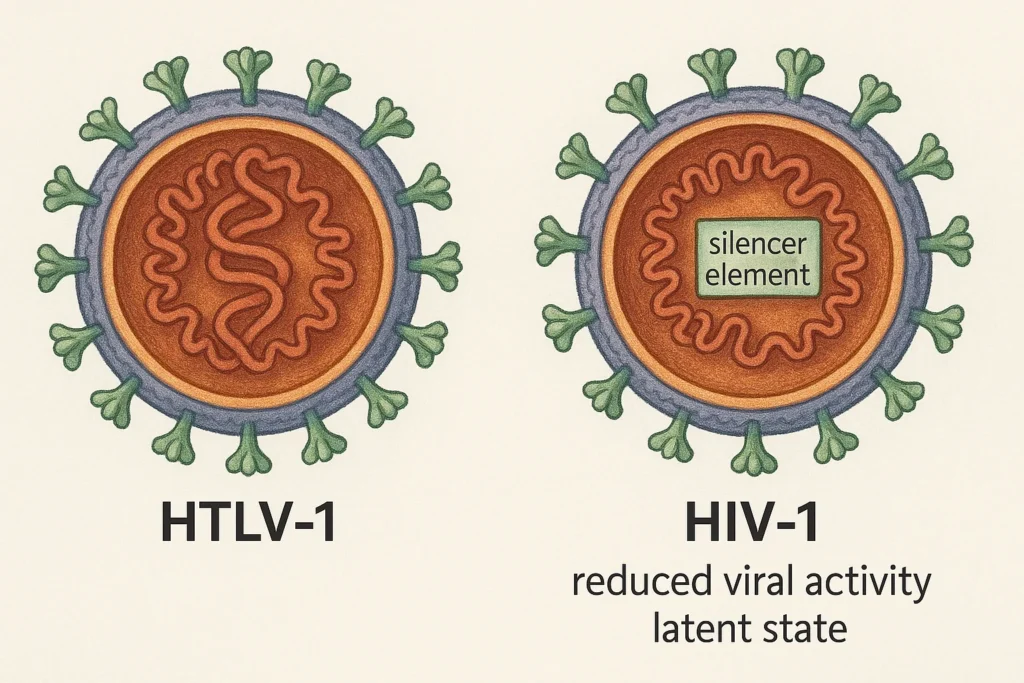
HTLV-1 vs HIV-1: The same family, the different strategies
Both HTLV-1 and HIV-1 will be human retroviruses with the ability to incorporate their genome into the DNA of the host. However there is a radical difference in their pattern of infection:
HIV-1 brutally spreads by creating excessive amounts of the virus in the blood and ultimately killing the immune system.
HTLV-1 will prefer to remain hidden and spread without creating additional virus by duplicating host cells.
The major benefit to HTLV-1? Latency. It evades immune responses and can stay in the host due to its ability to reduce the number of viral proteins presented on the surface of infected cells, hence, enabling it to life to decades. So far, scientists were unaware of the entire mechanism of the molecular switch that regulates such a strategy of hide and seek.
The Identification of the intragenic silencer element
With the help of sophisticated genomic techniques such as ATAC-seq (which allows identifying open areas on DNA) and ChIP-seq (which can define where proteins interact with DNA) researchers studied the genome of HTLV-1 on infected human blood cells. They discovered an unfamiliar open chromatin region (OCR) which was located in the viral pol gene.
This OCR did not merely serve as ornamentation; it served as a viral transcription silencer of the primary promoter of the virus, the 5 5 -LTR. Literally put, you have an inbuilt mute button of viral production.
RUNX1: The accomplice of the Virus
The team found that the silencer is functional due to the recruitment of the host protein RUNX1, co-regulators such as CBFNE and HDAC3. Under normal circumstances, these proteins are used to regulate the genes in a human being, yet HTLV-l steals them to maintain its genes turned off.
In the case of binding of RUNX1 to the silencer:
There was a precipitous reduction of viral gene expression.
Levels of the immune-visible viral protein Tax remained low.
The virus had a fixed dilatory concealed condition amidst the T cells.
By mutating the RUNX1-binding sites in the silencer, they increased the production of the virus-which increased visibility to the immune system.
Reason Why This Is Important in Immune Evasion
Latent infection of HTLV-1 is a juggling. In case of excessive replication of the virus, there are chances that infected cells can be recognized and killed by the immune system. This unfavorable side effect is prevented by using the silencer element to ensure that Tax expression is kept to a minimal. That is significant since Tax is one of the main targets of cytotoxic T lymphocytes (CTLs).
Interestingly, researchers were found out that by blocking RUNX1 with chemical inhibitor, production of Tax was enhanced and infected cells were found to be more susceptible to immune action. It might translate into future therapeutic potentials to control the interaction of RUNX1 silencers.
The HIV-1 Silencer Borrowing the Silencer for HIV-1
In order to find out whether this silencer has the potential to be relevant outside of HTLV-1, the team placed it into an HIV-1 genome. The findings were dramatic:
The production of HIV-1 drastically reduced.
The virus was more comparable to that of the HTLV-1 since it went into a latent-like state.
There was a reduction of adverse effects of HIV-1 on infected cells.
The experiment demonstrates how the silencer has an overwhelming ability of suppression, increasing the hope of engineering similar sequences in order to control other persistent viruses.
A New Model of Latent HTLV-1
The researchers suggest that in the model:
The silencer component in between the 5-LTR and viral insulator binds RUNX1 and collaborators.
This complex retains epigenetic modifiers that stalls chromatin in a closed state that they cannot be transcribed.
The virus up-regulates the expression of HBZ on the antisense strand, promoting the survival of the infected cells and remaining dormant.
Partial removal of the silencing may divert any transcriptional burst due to stimulation of the environment or the immune system, and the silencer returns latency.
Therapeutic Implications
Excavation of this mechanism of silencer may be the opening of a number of doors:
In the case of HTLV-1: The silencer can be targeted and which will “shock” the virus after which the infected cells can be visible to the immune system.
In the case of HIV-1: A similar application would be useful in controlling the viral production/restraining the destruction of the immune system by introducing similar silencers.
In a general virology sense: This is a fish print out of how small genetic switches can switch up infection results drastically.
Conclusion
HTLV-1 managed to infect humans since thousands of years and its part of the success is that it managed to remain incognito. It is a masterstroke by the virus in ensuring that it survives, through this new element in silencing, in co-operation with the host own RUNX1 protein.
The answer to a decades old puzzle by unmasking this mechanism shows scientists are not only revealing a way to fight HTLV-1 but is also pointing towards new ways to fight other chronic virus, as well.
The paper serves as a reminder of the fact that the genes that do not make the virus sometimes are the most powerful weapon in the viral arsenal.
References & Resources:
Sugata, K., Rahman, A., Niimura, K., et al. Intragenic viral silencer element regulates HTLV-1 latency via RUNX complex recruitment. Nature Microbiology, 10, 1447–1462 (2025). DOI:10.1038/s41564-025-02006-7
Centers for Disease Control and Prevention (CDC) – HTLV-1 Information
National Institute of Allergy and Infectious Diseases – HIV Basics