One of the most malignant cancers, pancreatic ductal adenocarcinoma (PDAC) has a dense tumor microenvironment (TME) that is resistant to the standard treatments. Recently there is a study published in Cancer Cell by Zhang et al. (2025) that shows a breakthrough option of targeting macrophocytosis, which is cellular process that among cancer cells and neighboring fibroblasts allowing survival under nutrient stress.
This study proves that by reducing macrophocytosis, cancer-related fibroblasts (CAFs) can be reprogrammed to improve the sensitivity of tumors to immunotherapy and chemotherapy. That is what you need to know about this promising discovery.
What is Macrophocytosis and Why does it Matter Pancreatic Cancer?
The process called macrophocytosis entails the capture of extracellular fluid and proteins as alternative sources of nutrition by cells, particularly those located in an environment that lacks nutrients. Pancratic cancer In pancreatic cancer:
CAFs as well as tumor cells depend on macrophocytosis to salvage proteins in cases of glutamine shortage (one of the essential nutrients).
Interfering with this process interferes with the metabolism of tumors and the effects of CAFs that significantly contribute to the resistance increase of tumors.
Related to the Study Key Findings
CAF Identity Is Supported by Macrophocytosis Under Stress
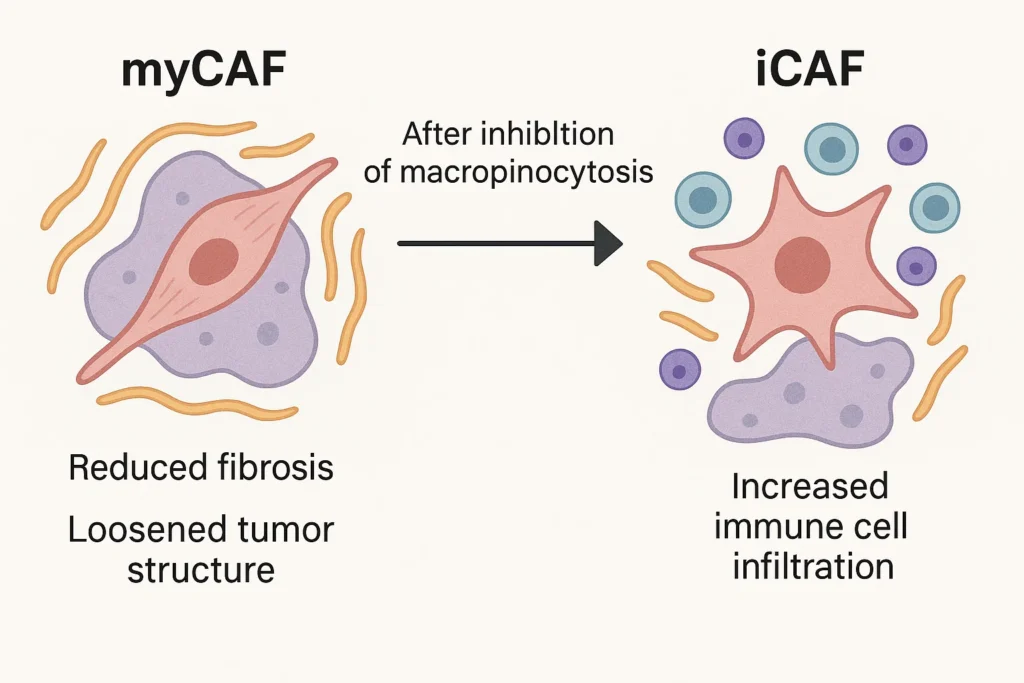
There are two subtypes of CAF:
myCAFs (myofibroblasts): enhance cancer fibrosis and hardening of the tumor to obstruct drug delivery.
iCAFs (inflammatory CAFs): Release cytokines which have an immunomodulatory effect.
In glutamine-depleted settings, inhibition of macrophocytosis induces the differentiation of myCAFs towards an iCAF-like phenotype with decreases in fibrosis and enhanced immune cell infiltration.
An inflammatory Switch is activated by Metabolic Stress
In conditions of glutamine deficiency when macrophocytosis is not allowed to take place, CAFs trigger MEK/ERK signaling that subsequently:
Production of proinflammatory cytokines (e.g., IL-6, CXCL1) in greater amounts.
Dysfunctional collagen production, which loosens up the tumor structure.
Improved Immune Response/Drug Delivery
Greater T-cell infiltration: Immunosuppressive macrophocytosis prevents CD4 + and CD8 + T cell in tumors.
Improved chemotherapy penetration: Drugs such as gemcitabine have a greater ease of access to cancerous tissues due to reduction of collagen.
Immunotherapy compatibility: The combination of the inhibition of macrophocytosis with anti-PD-1 therapy decreases metastasis.
Why This is Important in Pancreatic Cancers Therapy
PDAC is a thrifiily bad cancer to treat with its fibrotic tumor barrier and immune-suppressed tumor microenvironment. In this study, it is demonstrated that:
Drug delivery is enhanced due to the remodeling (decreased fibrosis) of the stroma.
Tumors that have their immune systems activated are more sensitive to checkpoint inhibitors.
Cancer cell survival is weakened by metabolic targeting (inhibition of macrophocytosis).
Why This Discovery Matters
PDAC’s desmoplastic TME resists treatments. Targeting CAF adaptations offers a breakthrough. By inducing myCAF-to-iCAF shifts, we exploit plasticity for antitumor effects.
Limitations: Mouse models; human translation needed. Safety of macropinocytosis inhibitors (like EIPA, not clinically approved) requires testing.
Future: Combine with existing drugs; explore in other fibrotic cancers.
For patients: Early detection key; discuss metabolic therapies with oncologists.
This research, funded by NIH and others, highlights metabolism’s role in cancer stroma—paving ways for personalized treatments.
Future Directions
Although the compound EIPA (a macrophocytosis inhibitor) was treated in this incidence, the future use of synthetically discovered more specific drugs that would be effective against this pathway may result in novel combination therapies. Clinical trials can possibly investigate:
Immunotherapy (anti-PD-1) + macrophocytosis inhibitors.
Stromal-targeting agents in order to potentiate chemotherapy.
Conclusion
This study brings out macrophocytosis as a major metabolic weakness in pancreatic cancer. Through reprogramming of CAFs, scientists may tone down the tumor fortifications, worsen immunity responses and drug outcome. Although additional research is required, this method may change the treatment of PDAC down the line.
References & Resources
Zhang Y, Ling L, Murad R, et al. (2025). Macropinocytosis maintains CAF subtype identity under metabolic stress in pancreatic cancer. Cancer Cell, 43, 1–20. https://doi.org/10.1016/j.ccell.2025.06.021
Sanford Burnham Prebys Medical Discovery Institute
National Cancer Institute – Immunotherapy for Pancreatic Cancer

