Heart disease remains a leading cause of death worldwide, with complex underlying mechanisms that challenge researchers and drug developers alike. Recent advances in stem cell biology and tissue engineering have given rise to human cardiac organoids (hCOs) miniature, simplified versions of the human heart grown in the lab. However, until recently, these organoids have lacked the maturity needed to faithfully replicate adult heart physiology and disease states. A groundbreaking study published in Nature Cardiovascular Research in 2025 reveals a novel maturation protocol for hCOs that unlocks their potential for complex disease modeling and drug discovery.
What Are Human Cardiac Organoids?
Human cardiac organoids are 3D clusters of heart cells derived from human pluripotent stem (hPS) cells. They self-organize into structures mimicking heart tissue, including cardiomyocytes (heart muscle cells), endothelial cells, fibroblasts, and other cell types. These organoids provide a versatile platform to study heart biology, test drug safety and efficacy, and model cardiovascular diseases in a controlled laboratory setting.
The Challenge of Maturation
A major limitation of existing hCOs is their immature state. Traditional protocols produce cardiomyocytes resembling fetal heart cells rather than fully mature adult heart cells, limiting their ability to model adult heart disease accurately or predict drug responses reliably. Extended culture times do not solve this problem, indicating that critical physiological cues are missing in conventional methods.
Breakthrough: Directed Maturation Protocol
The study introduces a novel Directed Maturation (DM) protocol that significantly advances the maturation of hCOs. The approach transiently activates two key molecular pathways:
5 AMP activated protein kinase (AMPK) a central regulator of cellular energy metabolism.
Estrogen related receptor (ERR) a transcription factor involved in mitochondrial and metabolic gene regulation.
By combining agonists for AMPK and ERR during a critical window in organoid development, researchers achieved enhanced expression of mature sarcomeric proteins and oxidative phosphorylation components hallmarks of adult cardiomyocyte function. This protocol increased the metabolic capacity and contractile force of hCOs and reduced their spontaneous contraction rate, indicating functional maturity closer to adult human heart tissue.
Cellular Complexity Mimicking the Human Heart
The DM-hCOs contain multiple cardiac cell types organized similarly to native human heart tissue, including cardiomyocytes, fibroblasts, endothelial cells, and epicardial cells. Single nuclei RNA sequencing confirmed the presence of key cardiac subtypes and paracrine signaling pathways that maintain cell populations without the need for continuous external growth factors.
Functional Advances in DM-hCOs
Improved Contractility and Metabolism: DM-hCOs showed increased cardiac troponin I (cTnI) expression, enhanced force of contraction, and higher mitochondrial respiratory capacity.
Stable Electrical Activity: The protocol yielded organoids with reduced spontaneous contraction rates and increased stability, crucial for reliable drug testing.
Enhanced Sarcoplasmic Reticulum Function: DM-hCOs demonstrated improved calcium handling, a key factor for synchronized heart muscle contraction.
Modeling Cardiac Diseases with DM-hCOs
One of the most exciting applications is the ability to model complex heart diseases. The study used DM-hCOs derived from stem cells harboring mutations in key cardiac proteins, such as:
Calsequestrin 2 (CASQ2) and Ryanodine Receptor 2 (RYR2): Mutations in these proteins cause arrhythmias. DM-hCOs displayed pro arrhythmic phenotypes mirroring clinical disease.
Desmoplakin (DSP) Mutation: DSP mutations lead to cardiomyopathies characterized by fibrosis and cardiac dysfunction. DM-hCOs with DSP mutations exhibited diastolic dysfunction and fibrosis-like features. This model helped identify a potential therapeutic compound, INCB054329, capable of mitigating the disease phenotype.
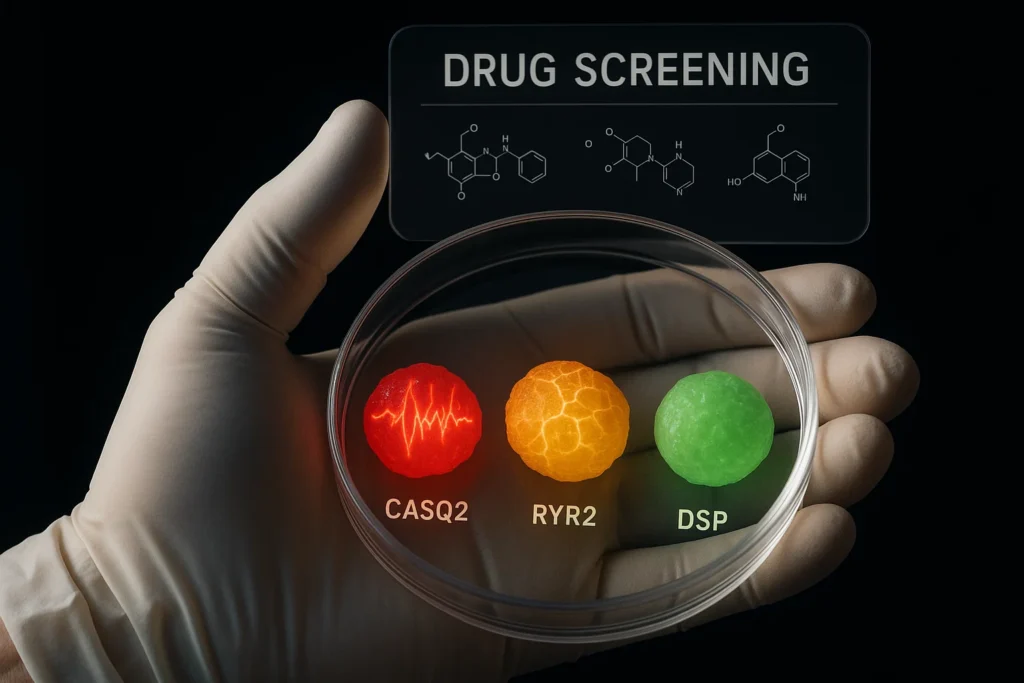
Drug Screening and Safety Testing
DM hCOs were tested with a panel of cardioactive drugs, including compounds known to affect heart rhythm and contractility. They reliably distinguished between high risk, intermediate risk, and low risk arrhythmogenic drugs, aligning well with clinical outcomes. Importantly, DM hCOs were sensitive to both inotropic agents (affecting contractile force) and agents influencing heart rate, making them a powerful platform for preclinical cardiac safety assessments.
Why This Matters
This new maturation method addresses a longstanding barrier in cardiac organoid technology the inability to replicate adult heart physiology in vitro. By providing mature, metabolically active, and multicellular cardiac models, researchers and pharmaceutical companies can:
Better understand mechanisms of heart diseases at the cellular level.
Develop personalized medicine approaches by modeling patientspecific mutations.
Accelerate drug discovery and improve safety profiling, potentially reducing costly late stage clinical failures.
Future Directions
The study highlights the promise of hCOs for translational cardiovascular research but also notes areas for further improvement, such as enhancing electrical pacing compatibility and extending maturation timelines. Integration with microfluidics and bioengineering could further refine these organoids as predictive models of human heart function.
References and Resources
Mills et al., Maturation of human cardiac organoids enables complex disease modeling and drug discovery, Nature Cardiovascular Research, 2025. DOI:10.1038/s44161-025-00669-3
National Center for Biotechnology Information (NCBI) – GSE156707
BioRender – BioRender.com
Related cardiac research on AMPK and ERR pathways