Cognitive decline is one of the personal and social issues as the world is getting older. Although age-related memory loss and a decline in mental capacity are relatively common phenomena, there is gaps in knowledge when it comes to the underlying molecular factors. Protein misfolding, which is the feature of many neurodegenerative diseases, is emerging as the major contributor.
Another innovative research study in 2025 by Tarbox et al. found its way to study minute, soluble misfolding of proteins within the hippocampus, an important structure involved in learning and memory, within aged rats through advanced proteomic procedure. The study throws some light on the potentially age-related changes in proteins structure that are required to cause cognitive decay in old age, not only the widely known aggregates of amyloid.
What the Study examined
The authors employed an age-advanced outbred rat model of 24 months old experimental animals the humoured proportion to old age of human beings. These rats were behaviorally examined, through the maze water of Morris, on their spatial memory and put as either:
Aged Unimpaired (AU): Rats who did not decline in their cognitive ability as compared to young adults
Aged Impaired (AI): Significantly impaired memory in rats
To find out what changes the protein structure had in response to them, the team isolated the hippocampus, and subjected it to Limited Proteolysis Mass Spectrometry (LiP-MS); a method that identifies alterations to protein structure based on its vulnerability toward enzymatic scission. They compared AU rats and AI rats, and through this, they found out those proteins with Cognition-Associated Structural changes (CASC) levels- i.e. those proteins with structural modifications associated with cognitive impairment.
Key Findings: Widespread Structural Changes in Soluble Proteins
214 proteins were identified with CASCs in the CA1 hippocampal subfield, about 8% of the proteins studied.
These structural changes were distinct from changes in protein abundance, which were minimal, indicating that protein function may be disrupted by conformation rather than quantity.
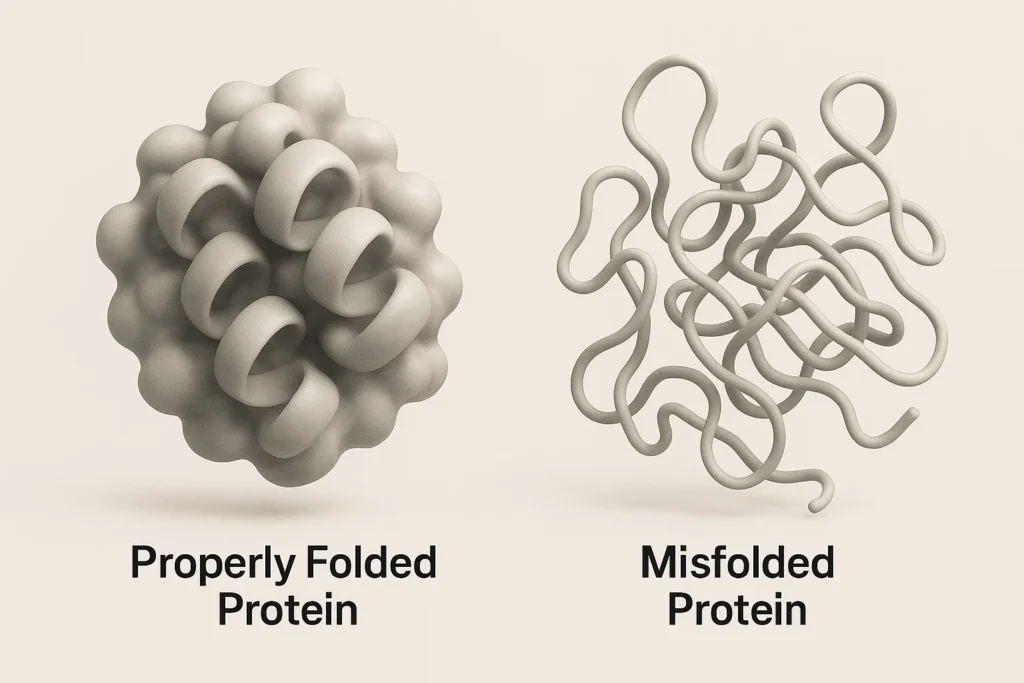
CASC proteins tended to be nonrefoldable—meaning they lacked the ability to spontaneously regain their normal shape after denaturation, making them more susceptible to lasting misfolding.
These proteins were soluble, unlike insoluble amyloid aggregates traditionally linked to neurodegeneration, suggesting that soluble misfolded proteins may also impair cognitive function.
Protein Paralysis Relevance and Cognitive Health
Protein folding occurs as a highly coordinated process in the cell and is facilitated by the proteostasis network the proteins can adopt the proper structure and thereby perform their functions properly. With age, this system gets derailed resulting in the build-up of misfolded proteins.
In the refolding experiments of the study, the difference with proteins containing CASCs showed a decreased ability to correct the mistakes during the protein folding results. Such inability to regain normal structure weakens the ability to refold and untangle the proteins which are out of shape leading to their buildup possibly causing disturbance in cell performance and loss of cognition.
Proteins Linked to Neurodegeneration and Memory
The researchers noted a number of proteins among the CASCs that have already been hinted at in work with the processes of thought and with degenerative diseases of the nervous system:
Glutathione s-transferase Lancl1: Resistance to oxidative stress of neurons.
Ubiquitin-conjugating enzyme E2 D3: A component of protein degradation system.
Superoxide dismutase (SOD2): It is a mitochondrial enzyme that guards the cells against oxidative damage.
ATP dependent 6-phosphofructokinase (PFKL): Important glycolytic enzyme, metabolic abnormalities are typical of aging brains.
V-type proton ATPase subunit (Atp6v0c): This is a component of synaptic vesicle loading and autophagy.
Alteration of the structure in these proteins can affect the survivability of neurons, energy metabolism, and synaptic functioning which are very essential in memory formation.
Broader Pathways Affected: Protein Degradation and Synaptic Function
CASCs were found enriched in proteins involved in the ubiquitin-proteasome system (UPS), which regulates protein degradation. Dysfunction in UPS has been linked to aging and neurodegeneration, and structural changes in UPS proteins may impair clearance of damaged proteins.
Additionally, proteins at the synapse, such as gephyrin and 14-3-3 proteins, also showed structural changes. These proteins regulate synaptic plasticity and neurotransmitter receptors, suggesting that misfolding here might disrupt neuronal communication.
Different Than Post-Translational Modifications
The study has also eliminated the possibility with the help of a careful study that the observed structural changes can be caused by a difference in post-translational modifications (PTMs) including phosphorylation or oxidation. It reinforces the idea that it is not solely modifications of the chemical level on which these CASCs are based.
What Does This Mean for Understanding Cognitive Decline?
The presence of a high abundance of soluble, misfolded proteins with aberrant refolding discovered in cognitively impaired aged rats is indicative of a wide-spread molecular process as separate to the conventional amyloid aggregation.
This highlights novel therapeutic targets, proteins any of which might have a controlling value in holding its structure stable so as to maintain mental performance, and also to new prognostic biomarkers, and these not in terms of abundance but of protein structure.
Future Directions and Challenges
Although the study identifies major changes in structure, it does not so far establish how these changes come to being and either explicitly or none explicitly affect the protein functioning. There is more work to be done:
Confirm CASC proteins as etiological factors of cognitive decline.
Investigate the role of molecular chaperones and place in proteostasis that are altered by aging.
Identify drugs that will refold proteins normally or accelerate the removal of misfolded proteins.
Explore the connection between poor protein turnover and such structural changes.
Conclusion: Toward a Molecular Understanding of Brain Aging
The study by Tarbox et al. has offered a strong new glimpse of the molecular perspective on cognitive aging. It disrupts the standard emphasis on amyloid plaques by showing that soluble hippocampal proteins are subtly and pervasively misfolded in a process associated with memory loss and newly enables research and intervention.
The key to keeping our memories and mental functions healthy may lie in the understanding and addressing the abnormalities in protein folding.
Reference:
Tarbox, H.E., Branch, A., & Fried, S.D. (2025). Proteins with cognition-associated structural changes in a rat model of aging exhibit reduced refolding capacity. Science Advances, 11, eadt3778.