In the past few decades, the treatment of diabetes type 2 came to be a lot about managing blood sugar levels. However, what would happen should the key to it all on how to avoid this complicated disease and how to comprehend it be hidden deep within our muscles? The first study in the influential journal Cell shows a groundbreaking map in our body, a cellular fingerprint that surprisingly accurately anticipates the tendency to become insulin-resistant and become diabetic type 2. This is not only about diagnosis; it is about opening the doors to really personalized treatments.
The Big Reveal: The Molecular Imprint of Your Muscle
The researchers studied muscle biopsy following 120 men and women, in whom blood sugar was either normal or type 2 diabetes, yet to differing levels of insulin sensitivity (how successfully the cells reacted to the insulin). Then with advanced technology known as proteomics, and phosphoproteomics; they mapped thousands of proteins and phosphorylation sites (small molecular switches that turn genes on or off) in the skeletal muscle of each individual.
The ground breaking discovery? The strength of the correlation between your whole-body insulin sensitivity and the molecular landscape of your muscle before stimulation with insulin known as fasting. Specifically:
Fasting Muscle as a Crystal Ball: The distribution of proteins and phosphosites (“phosphorylation sites”) within the resting muscle had a very close correlation to the degree of insulin-sensitivity or resistance of a person: i.e. what the muscle looked like at rest was very closely connected with insulin-sensitive or insulin-resistant. This signature of fasting outperformed the conventional product classifications (such as diabetic or non-diabetic) and even as well as fasting blood sugar sensitivities in predicting the insulin resistance.
The A Protagonist in Resistance: In a single phosphorlang return, S65 on the AMPK-gamma-3 protein (AMPK-gamma-3 S65) was the most spectacular. The amount of this particular phosphosite was negatively correlated with insulin sensitivity; that is, the more phosphorylated a person was, the more resistant he/she was. Interestingly, the site is human specific, and it is constrained by stress/inflammation signaling (p38/MAPKAPK2). Perturbation of muscle cell AMPK in which levels of the isoform AMPK3 were reduced actually enhanced insulin action, indicating this pathway represents a therapeutic target.
Insulin Signaling Is Not an All or None Issue: Contrary to what has been believed for a long time the study found that insulin resistance did not cripple all the insulin signaling pathways similarly.
Persistent Signalling: The critical downstream effects of insulin signalling, directly downstream, especially via the resulting protein AKT (e.g. the phosphorylation of TBC1D4 T642), were intact surprisingly well even in individuals with the worst cases of insulin resistance. Insulin would have the potential to effectively switch these switches.
Dysregulated Pathways: Nonetheless, signalling further downstream, especially in relation to the nutrient-sensor mTOR (e.g. phosphorylation of RPTOR S863 and EIF4EBP1 T70) was profoundly impaired in insulin resistance. This emphasizes the term selective insulin resistance -experiments show that some pathways are deactivated and others are ordinary.
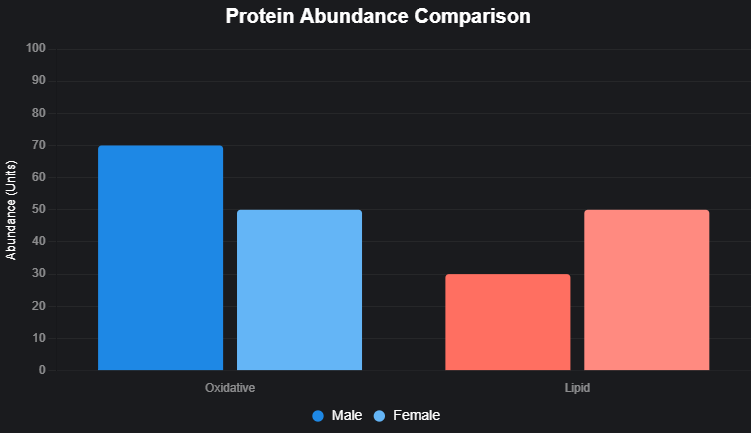
Matters of Metabolic Machinery: The overall abundance of the cellular energy production sites (the mitochondria or the power plants), the ratio of proteins in the process of burning sugars (glycolysis) versus burning fat and oxygen (oxidative metabolism) in the muscle was also strongly connected to insulin sensitivity. Individuals who were insulin sensitive possessed more mitochondrial proteins and proportion of those more oriented to oxidative metabolism.
Insulin Resistance Biology Described by Sex: Although the patterns of the muscle proteome and phosphoproteome appeared quite different in men and women (e.g., for fat handling the women had more proteins than men, the reverse was true of sugar metabolism), the fundamentals of the molecular markers of insulin resistance per se were strikingly similar between the sexes. The pathogenic processes seem to be preserved.
The reason this research is a game-changer
Change in the FOCUS: It changes the FOCUS away only on blood glucose and on HbA1c to tissue dysfunction (muscle insulin resistance) much earlier, which can help intervene earlier.
Personal Medicine Possibility: The possibilities include development of specific molecular signatures (such as high AMPKgamma3 S65 phosphorylation or low levels of mitochondrial proteins) against which doctors can group people by their individual biomolecular background, then develop their prevention or treatment regimen. The single approach of diabetes has become out-dated.
New Drug Targets: With the identification of AMPK S65 and the validation of maladjusted mTOR response, it is possible to have such specific targets of drugs that are developed to enhance muscle insulin resistance.
Patterns of Complexity: The discovery of selective insulin resistance (spared AKT, defective mTOR) was able to explain several inconsistencies in the body of literature, and can provide a more precise answer to why insulin action fails.
Beyond Diagnosis: The study highlights the fact that insulin sensitivity is continuous, and heavy variations within molecules exist despite even defining diagnostic group (NGT or T2D). The health of our muscles highlighted by its molecular composition is essential.
The Future: Future Of Muscle Maps to Personalized Health
With this study, a future can be opened where:
A muscle biopsy (or possibly a blood test based on these signatures) would be able to measure your individual risk of becoming insulin resistant many years before the level of blood sugar increases.
Your unique molecular defect identified in muscle could be treated by prescription of lifestyle measures by doctors or drugs at a specific molecular level (e.g., mitochondrial boosting, AMPK S65 phosphorylation inhibitor or mTOR ).
New diabetes drugs that will be able to treat the disease could be tested during clinical trials where each participant will be stratified according to the presence of different frames of the muscles, increasing successful results in the treatment of diabetes.
Conclusion
This seminal research discloses that our skeletal muscle contains an extensive map of molecules- a proteomic and phosphoproteomic pattern of our skeletal muscle that has high predictive value against insulin resistance which is the origin of type 2 diabetes. By transcending to more elaborate measurements of blood sugar and by going inside the molecule at the molecular machine of the muscles, scientists have unearthed some crucial new information, namely the predictive utility of the fasting state, the functionality of the players in the game such as AMPKgamma3 S65 and mTOR as well as the concept of selective insulin resistance and the basic similarity of the resistance mechanism in both men and women. The research is not only concerned with making diabetes more comprehensible, but it gives the basis of developing a future of truly individualized medicine, one that allows the prevention and treatment to be targeted to the individual biological landscape lying within the muscles of individuals, and that gives hope of formulating more successful measures to overcome this worldwide health condition.
Reference
Kjaergaard et al. (2025)
Personalized molecular signatures of insulin resistance and type 2 diabetes
Journal: Cell 188, 1–17
DOI: https://doi.org/10.1016/j.cell.2025.05.005